What is creatine?
The use of creatine (Cr) can be traced back to the early 1990s when several elite sprint athletes reported performance-enhancing benefits following gold medal winning performances at the 1992 Barcelona Olympic Games (Anderson, 1993). This sparked the birth of a new era with creatine gaining widespread popularity as a legitimate ergogenic aid (Bird, 2003). Creatine is a nitrogenous organic acid abundant in metabolically active muscle, heart and brain tissue. It is synthesised endogenously in the liver and kidneys from the amino acids arginine, glycine and methionine, and absorbed from the diet primarily from red and white meat (Chilibeck et al., 2017; Phillips, 2015). Most creatine is stored intramuscularly as phosphocreatine (PCr) (Candow et al., 2014). PCr functions principally as a temporal energy buffer by donating a high-energy phosphate to ADP through the enzymatic reaction of creatine kinase, which re-synthesises and replenishes ATP stores and thus helps maintain skeletal muscle energy availability during very short, intense anaerobic exercise (Kreider et al.,, 2017; Candow et al., 2014; Candow & Chillibeck, 2010). PCr also acts as a spatial energy buffer shuttling intracellular energy between mitochondria and sites of cellular ATP utilization (Kreider et al., 2017; Gualano et al., 2016).

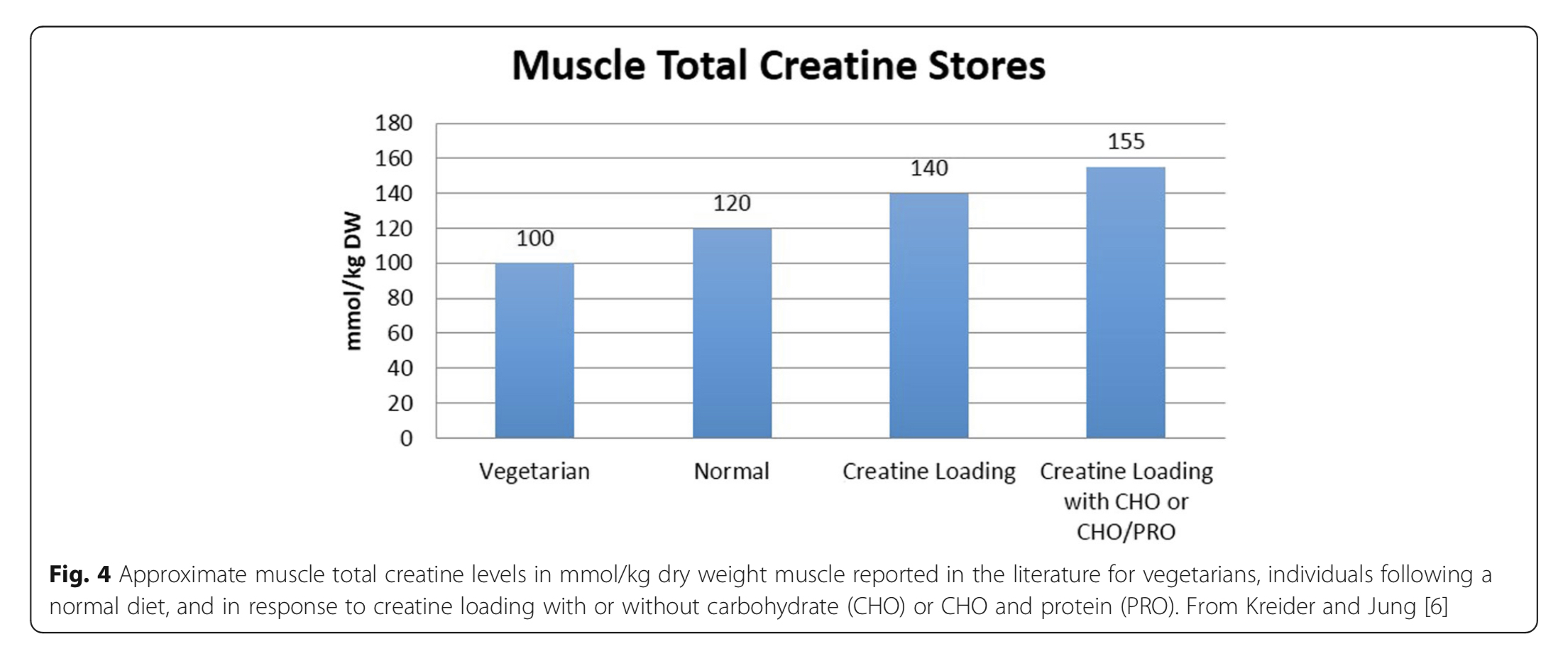
Creatine supplementation increases the Cr/PCr reservoir by 20-40% (Kreider et al., 2017) and it is posited that this enhances PCr-mediated ATP resynthesis during and after high-intensity exercise bouts (Deane et al., 2017; Close et al., 2016), thereby allowing greater amounts of work to be accomplished (Phillips, 2015). This is particularly relevant for resistance training (RT) given that a dose-response relationship has been shown to exist between training volume and gains in skeletal muscle mass (Schoenfeld et al., 2017a; Schoenfeld et al., 2017b), and muscle strength (Figueiredo et al., 2017; Ralston et al., 2017). Other possible mechanisms to account for creatine ergogenicity are reduced exercise-induced muscle damage, reduced oxidative stress, increased GLUT4 in muscle fibre membranes, increased cell swelling that activates protein synthesis within muscle fibres, and decreased reliance on anaerobic glycolysis/reduced lactate production (Chilibeck et al., 2017; Kreider et al., 2017; Devries and Phillips, 2014). Although the exact mechanisms of action are still to be determined (Phillips, 2015), creatine supplemented RT has been extensively researched, especially in younger populations (Buford et al., 2007; Kreider et al., 2017). Consuming 5 grams of creatine (or 0.3 grams per kilogram body weight) four times daily for 5-7 days is generally viewed as the most effective way to increase muscle creatine stores and can be adequately maintained by consuming 3-5 grams/day following this loading phase (Kreider et al., 2017).
Is creatine effective in older adults?
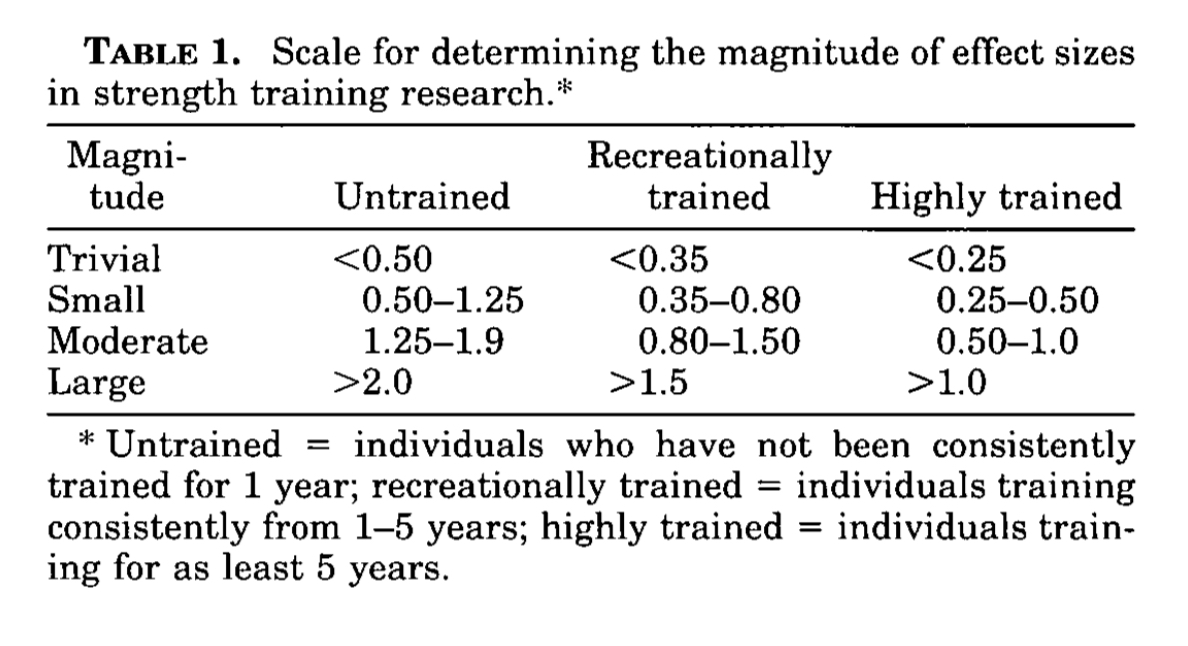
Three meta-analyses of randomised, placebo-controlled trials (Chilibeck et al., 2017; Candow et al., 2014; Devries and Phillips, 2014) have been published on the effects of creatine supplementation during RT on lean tissue mass and muscle strength in middle-aged and older adults (45-80 years old). No meta-analysis has yet assessed the effectiveness of creatine on RT outcomes in adults specifically 60 years or older. Most recently, Chilibeck et al. (2017, pg219) found significantly greater increases in lean tissue mass (1.4 kg; SMD=1.35), upper (i.e. chest press; SMD=0.37) and lower (i.e. leg press; SMD=0.25) body muscle strength when middle-aged to older adults (50-80 years old) were supplemented with creatine during RT. However, whilst the results of this study are often promoted as evidence that creatine has ergogenic value for this cohort, the standardised mean differences reported for muscle strength were trivial based on the effect sizes proposed by Rhea (2004) to delineate what is, and what is not meaningful following RT. If we apply more traditional effect sizes (Sullivan and Feinn, 2012), these strength improvements still remain small. One of the longest trials to investigate the impact of creatine supplementation and RT in middle-aged and older male adults (49-69 years old) found no additional benefits on measures of bone, muscle or strength after 12 months (Candow et al., 2020). Moreover, Beaudart et al., (2018) concluded that the research findings were equivocal for creatine after conducting a systematic review into the effects of various nutrients on muscle mass, muscle strength and physical performance in older adults (≥60 years old).
At the time of writing only a handful of studies have assessed the monotherapy supplementation of creatine in older adults (≥60 years) undergoing RT (Smolarek et al., 2020; Gualano et al., 2014; Deacon et al., 2008; Pinto et al 2016; Aguiar et al., 2013; Alves et al., 2013; Brose et al 2003; Chrusch et al., 2001; Bermon et al., 1998). Of those that measured changes in lean tissue mass, participants supplemented with creatine consistently achieved greater benefits compared to placebo (Gualano et al., 2014; Pinto et al 2016; Aguiar et al., 2013; Brose et al 2003; Chrusch et al., 2001). In contrast, the effects of creatine on muscle strength were less consistent with the vast majority of studies showing no additional benefit versus placebo for core RT lower limb exercises i.e. leg press (Deacon et al., 2008; Pinto et al., 2016; Alves et al., 2013; Brose et al 2003; Bermon et al., 1998). Three of the four studies that explored the impact of creatine on physical function found no evidence of performance enhancement compared to placebo for a number of standard tests (Aguiar et al., 2013; Brose et al 2003; Gualano et al. 2014; Deacon et al., 2008); these included the 30-second chair stand test, 30 metre walk time, time to climb 14 stairs, the timed-up-and-go test, and the shuttle walk distance test. No RCTs have yet tested whether creatine supplementation in older adults (≥60 years) positively impacts balance or quality of life. Encouragingly though, Neves et al., (2011) demonstrated improved quality of life and physical function in postmenopausal women (mean age=58 years old) with knee osteoarthritis that took creatine during RT. The only study to have assessed the effect of creatine on dynamic balance found that improvement of balance performance was actually inhibited in older middle-aged adults (Johannsmeyer et al., 2016); those that were randomly allocated to the placebo group during drop-set RT experienced significantly greater improvement in dynamic balance (30.8%) compared to the creatine group (19.4%), with a reduction in balance errors detected for the placebo group only. In sum, creatine supplementation in older adults during RT appears to support increased lean tissue mass, but this has not necessarily translated into appreciable gains in muscle strength, physical function and/or improved balance versus placebo.
Is creatine well tolerated and safe?
Creatine when used in healthy, older adults appears to be well tolerated and safe. There have been no reports or evidence of any adverse effects that are serious in nature (Chilibeck et al., 2017; Goudarzian et al., 2017; Pinto et al., 2016; Gualano et al., 2014; Alves et al., 2013; Brose et al 2003; Chrusch et al., 2001; Bermon et al., 1998) and self-reported issues associated with the use of creatine have been uncommon (Pinto et al 2016; Gualano et al., 2014; Alves et al., 2013; Brose et al., 2003). No adverse events related to, nor changes in either kidney or liver function have been reported from RCTs (Gualano et al., 2014; Tarnopolsky et al., 2007; Brose et al., 2003) and other studies that included both middle-aged and older adults are devoid of any such side-effects (Johannsmeyer et al., 2016; Chilibeck et al., 2015; Lobo et al., 2015; Cornelissen et al., 2010; Eijnde et al., 2003). Some studies have reported that gastrointestinal (GI) distress and muscle cramping and/or muscle strain may be more common in those receiving creatine (Chilibeck et al., 2015; Chrusch et al., 2001). In healthy, older men (mean age=70 years old) loose stools were reported by Chrusch et al., (2001) as a side-effect during the 1-week loading phase and increased muscle cramping/strain occurred between weeks 3 and 5. Middle-aged to older postmenopausal women (mean age=57 years old) taking creatine experienced a higher number of these adverse events when GI complaints and muscle cramping were grouped for assessment (Chilibeck et al., 2015). None of these side-effects led to study discontinuation and appear to be transient in nature with no impairment of exercise training response noted.
What questions do we need further clarification on?
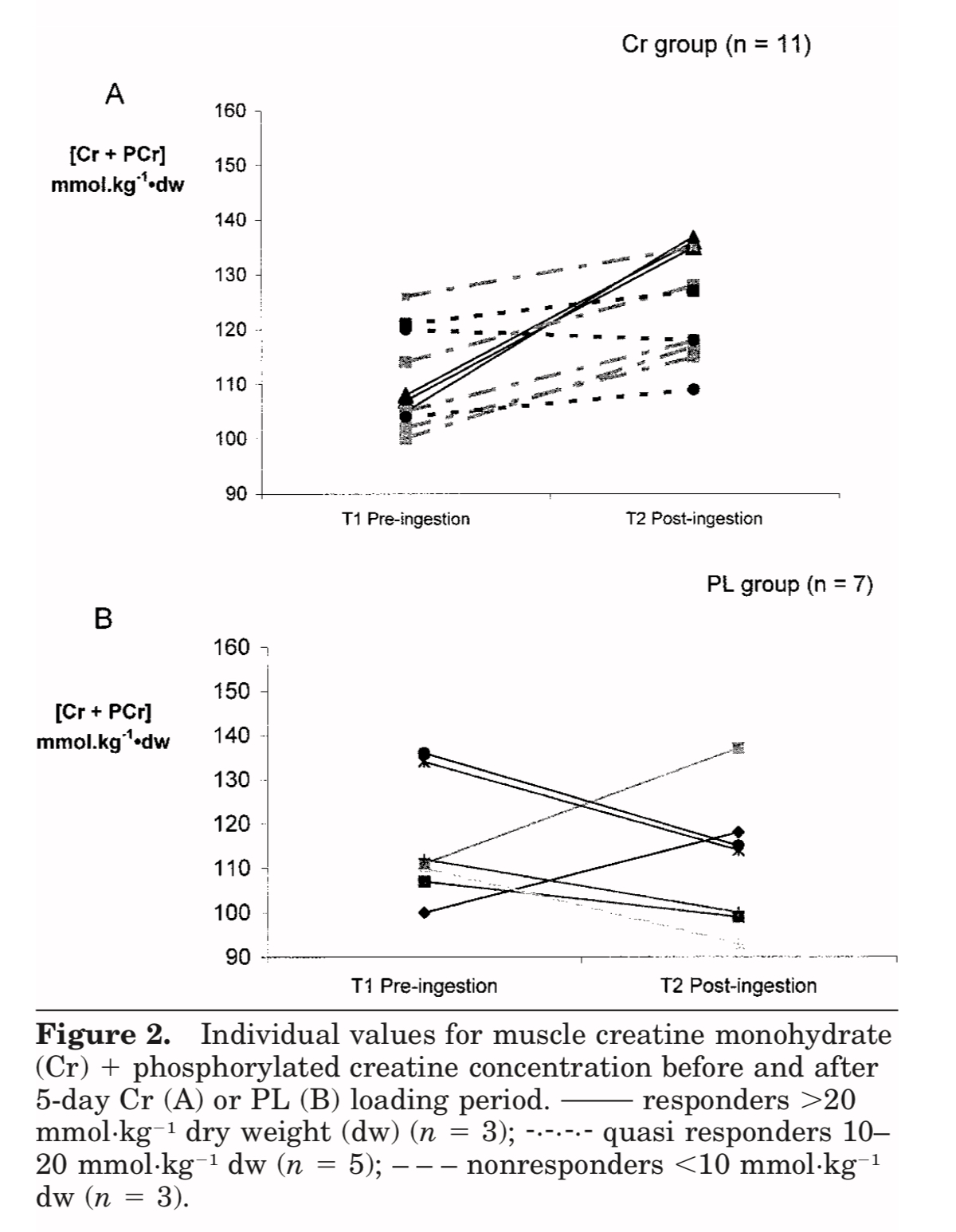
Many questions remain unresolved. Despite the evidence supporting increased lean tissue mass following creatine supplementation, it seems too early to claim definitively that such a strategy substantially and consistently improves muscle strength or physical function in all older adults undergoing RT. Training adaptations, in theory, should be augmented by creatine. It is well acknowledged that ageing causes skeletal muscle atrophy with disproportionately greater reduction in cross-sectional area (CSA) of PCr-rich type-II muscle fibres (Nilwik et al., 2013; Kushmerick et al., 1992), and this results in much lower levels of intramuscular creatine in the quadriceps vastus lateralis (thigh) muscle of older versus younger adults (Chilibeck et al., 2017). Lifestyle changes with ageing – particularly reduced dietary meat intakes, decreased physical activity levels (Chilibeck et al., 2017) and increased sedentary time (Diaz et al., 2017; Dunlop et al., 2015) – may further impact muscle PCr levels and modify any potential benefits of creatine supplementation. Further research is therefore required to establish whether the magnitude and heterogeneity of RT adaptations is modulated by significant inter-individual differences in creatine uptake kinetics, given that training responsiveness is correlated to the change in intramuscular creatine stores. Research by Syrotuik and Bell (2004) provide support for this possibility where it was demonstrated that young, healthy men had 3 different levels of response to a 5-day creatine load as measured by post-supplementation intramuscular creatine levels. Responders to creatine loading possessed a biological profile of the lowest initial muscle Cr/PCr levels, greatest percentage of type-II muscle fibres, largest muscle fibre CSA and lean tissue mass, plus were the only subjects to achieve improvement in 1RM leg press when compared to quasi- and non-responders. It is plausible that this may partially account for the lack of consistency in the research as such inter-individual variation could significantly water down any generalised group benefits. These responder profiles outlined above also raise another potential limitation in those older adults that are most in need of an ergogenic effect (i.e. those with sarcopenia and muscle weakness), as they may be the least likely, by extension, to reap the meaningful benefits of creatine supplementation. Furthermore, it is well accepted that intramuscular post-supplementation creatine levels (at day 28) are comparable for “slow” (3 grams/day for 1 month) and “rapid” load (4×5 grams/day for 5-7 days and 3-5 grams/day thereafter) protocols (Hultman et al., 1996). Thus, it would be prudent to compare whether the slow load approach is better tolerated than the rapid load approach (i.e. reduction of GI-related side-effects and muscle cramping/pulls) based on the evidence where some older adults appear to be more sensitive to large initiation doses of creatine.
Final comments
It is worth mentioning that besides any possible beneficial effect of creatine on lean tissue mass, skeletal muscle strength or function, there are several other therapeutic reasons that may potentially justify such use in older adults. An increasing amount of data from both human and rodent experiments support multiple other benefits for creatine, including lowering cholesterol and triglyceride levels, reducing liver fat accumulation, decreasing homocysteine levels, having antioxidant properties, improving glycaemic control, slowing tumor growth in some types of cancers, mitigating bone loss, positively affecting cognitive function and in some cases, serving as an antidepressant (Kreider et al., 2017). Consequently, the position stand on the safety and efficacy of creatine supplementation in exercise, sport, and medicine published by the International Society of Sports Nutrition concluded that (2017:11):
Available short and long-term studies in healthy and diseased populations, from infants to the elderly, at dosages ranging from 0.3 to 0.8 g/kg/day for up to 5 years have consistently shown that creatine supplementation poses no adverse health risks and may provide a number of health and performance benefits.
References
Aguiar AF, Januário RS, Junior RP, Gerage AM, Pina FL, Do Nascimento MA, Padovani CR, Cyrino ES. Long-term creatine supplementation improves muscular performance during resistance training in older women. European journal of applied physiology. 2013 Apr 1;113(4):987-96.
Alves CR, Merege Filho CA, Benatti FB, Brucki S, Pereira RM, de Sá Pinto AL, Lima FR, Roschel H, Gualano B. Creatine supplementation associated or not with strength training upon emotional and cognitive measures in older women: a randomized double-blind study. PLoS One. 2013 Oct 3;8(10):e76301.
Anderson O. (1993) Creatine propels British athletes to Olympic gold medals: Is creatine the one true ergogenic aid? Running Research News 9, 1-5
Beaudart C, Rabenda V, Simmons M, Geerinck A, Araujo DC, Reginster JY, Amuthavalli TJ, Bruyère O. Effects of Protein, Essential Amino Acids, B-Hydroxy B-Methylbutyrate, Creatine, Dehydroepiandrosterone and Fatty Acid Supplementation on Muscle Mass, Muscle Strength and Physical Performance in Older People Aged 60 Years and Over. A Systematic Review on the Literature. The journal of nutrition, health & aging. 2018;22(1):117.
Bermon S, Venembre P, Sachet C, Valour S, Dolisi C. Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Acta Physiologica Scandinavica. 1998 Oct 1;164:147-56.
Bird SP. Creatine Supplementation and Exercise Performance: A Brief Review. Journal of Sports Science & Medicine. 2003;2(4):123-132.
Brose A, Parise G, Tarnopolsky MA. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003 Jan 1;58(1):B11-9.
Buford TW, Kreider RB, Stout JR, Greenwood M, Campbell B, Spano M, Ziegenfuss T, Lopez H, Landis J, Antonio J. International Society of Sports Nutrition position stand: creatine supplementation and exercise. Journal of the International Society of Sports Nutrition. 2007 Aug 30;4(1):6.
Candow et al. Effect of 12 months of creatine supplementation and whole-body resistance training on measures of bone, muscle and strength in older males. Nutr Health. 2020, Nov 24 (online ahead of print)
Candow DG, Chilibeck PD, Forbes SC. Creatine supplementation and aging musculoskeletal health. Endocrine. 2014 Apr 1;45(3):354-61.
Candow DG, Chilibeck PD. Potential of creatine supplementation for improving aging bone health. The journal of nutrition, health & aging. 2010 Feb 1;14(2):149-53.
Candow DG, Zello GA, Ling B, Farthing JP, Chilibeck PD, McLeod K, Harris J, Johnson S. Comparison of creatine supplementation before versus after supervised resistance training in healthy older adults. Research in Sports Medicine. 2014 Jan 2;22(1):61-74.
Chilibeck PD, Candow DG, Landeryou T, Kaviani M, Paus-Jenssen L. Effects of creatine and resistance training on bone health in postmenopausal women. Medicine & Science in Sports & Exercise. 2015 Aug 1;47(8):1587-95.
Chilibeck PD, Kaviani M, Candow D, Zello, G. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access Journal of Sports Medicine 2017 Nov 2; 8: 213-226.
Chrusch MJ, Chilibeck PD, Chad KE, Davison KS, Burke DG. Creatine supplementation combined with resistance training in older men. Medicine & Science in Sports & Exercise. 2001 Dec 1;33(12):2111-7.
Close GL, Hamilton DL, Philp A, Burke LM, Morton JP. New strategies in sport nutrition to increase exercise performance. Free Radical Biology and Medicine. 2016 Sep 30;98:144-58.
Cornelissen VA, Defoor JG, Stevens A, Schepers D, Hespel P, Decramer M, Mortelmans L, Dobbels F, Vanhaecke J, Fagard RH, Vanhees L. Effect of creatine supplementation as a potential adjuvant therapy to exercise training in cardiac patients: a randomized controlled trial. Clinical rehabilitation. 2010 Nov;24(11):988-99.
Deane CS, Wilkinson DJ, Phillips BE, Smith K, Etheridge T, Atherton PJ. “Nutraceuticals” in relation to human skeletal muscle and exercise. American Journal of Physiology-Endocrinology and Metabolism. 2017 Apr 1;312(4):E282-99.
Devries MC, Phillips SM. Creatine supplementation during resistance training in older adults-a meta-analysis. Med Sci Sports Exerc. 2014;46(6):1194–203
Diaz KM, Howard VJ, Hutto B, Colabianchi N, Vena JE, Safford MM, Blair SN, Hooker SP. Patterns of sedentary behavior and mortality in US Middle-aged and older adults: a national cohort study. Annals of internal medicine. 2017 Oct 3;167(7):465-75.
Dunlop DD, Song J, Arnston EK, Semanik PA, Lee J, Chang RW, Hootman JM. Sedentary time in US older adults associated with disability in activities of daily living independent of physical activity. Journal of physical activity & health. 2015 Jan;12(1):93.
Eijnde BO, Van Leemputte M, Goris M, Labarque V, Taes Y, Verbessem P, Vanhees L, Ramaekers M, Eynde BV, Van Schuylenbergh R, Dom R. Effects of creatine supplementation and exercise training on fitness in men 55–75 yr old. Journal of Applied Physiology. 2003 Aug 1;95(2):818-28.
Figueiredo VC, de Salles BF, Trajano GS. Volume for Muscle Hypertrophy and Health Outcomes: The Most Effective Variable in Resistance Training. Sports Medicine. 2017 Oct 11:1-7.
Goudarzian M, Rahimi M, Karimi N, Samadi A, Ajudani R, Sahaf R, Ghavi S. Mobility, Balance, and Muscle Strength Adaptations to Short-Term Whole Body Vibration Training Plus Oral Creatine Supplementation in Elderly Women. Asian Journal of Sports Medicine. 2017 Mar 1;8(1).
Gualano B, Macedo AR, Alves CR, Roschel H, Benatti FB, Takayama L, de Sá Pinto AL, Lima FR, Pereira RM. Creatine supplementation and resistance training in vulnerable older women: a randomized double-blind placebo-controlled clinical trial. Experimental gerontology. 2014 May 31;53:7-15.
Gualano B, Rawson ES, Candow DG, Chilibeck PD. Creatine supplementation in the aging population: effects on skeletal muscle, bone and brain. Amino acids. 2016 Aug 1;48(8):1793-805.
Hultman E, Soderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in men. Journal of applied physiology. 1996 Jul 1;81(1):232-7
Johannsmeyer S, Candow DG, Brahms CM, Michel D, Zello GA. Effect of creatine supplementation and drop-set resistance training in untrained aging adults. Experimental gerontology. 2016 Oct 31;83:112-9.
Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, Candow DG, Kleiner SM, Almada AL, Lopez HL. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. Journal of the International Society of Sports Nutrition. 2017 Jun 13;14(1):18.
Kuriansky J, Gurland B. The performance test of activities of daily living. The International Journal of Aging & Human Development.1976; 7:343-352.
Kushmerick MJ, Moerland TS, Wiseman RW. Mammalian skeletal muscle fibers distinguished by contents of phosphocreatine, ATP, and Pi. Proceedings of the National Academy of Sciences. 1992 Aug 15;89(16):7521-5
Lobo DM, Tritto AC, da Silva LR, de Oliveira PB, Benatti FB, Roschel H, Nieß B, Gualano B, Pereira RM. Effects of long-term low-dose dietary creatine supplementation in older women. Experimental gerontology. 2015 Oct 31;70:97-104.
Neves Jr M, Gualano B, Roschel H, Fuller R, Benatti FB, Pinto AL, Lima FR, Pereira RM, Lancha Jr AH, Bonfa E. Beneficial effect of creatine supplementation in knee osteoarthritis. Medicine and science in sports and exercise. 2011 Aug;43(8):1538-43.
Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, van Loon LJ. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Experimental gerontology. 2013 May 31;48(5):492-8.
Phillips SM. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Advances in Nutrition: An International Review Journal. 2015 Jul 1;6(4):452-60.
Pinto CL, Botelho PB, Carneiro JA, Mota JF. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. Journal of cachexia, sarcopenia and muscle. 2016 Sep 1;7(4):413-21.
Ralston GW, Kilgore L, Wyatt FB, Baker JS. The Effect of Weekly Set Volume on Strength Gain: A Meta-Analysis. Sports Medicine. 2017 Jul 28:1-7.
Rhea MR. Determining the magnitude of treatment effects in strength training research through the use of the effect size. Journal of strength and conditioning research. 2004 Nov 1;18:918-20.
Schoenfeld BJ, Ogborn D, Krieger JW. Dose-response relationship between weekly resistance training volume and increases in muscle mass: A systematic review and meta-analysis. Journal of sports sciences. 2017a Jun 3;35(11):1073-82.
Schoenfeld BJ, Ogborn D, Krieger JW. The dose–response relationship between resistance training volume and muscle hypertrophy: are there really still any doubts?. Journal of sports sciences. 2017b Oct 18;35(20):1985-7.
Sullivan, Gail M., and Richard Feinn. “Using effect size—or why the P value is not enough.” Journal of graduate medical education 4, no. 3 2012: 279-282.
Syrotuik DG, Bell GJ. Acute creatine monohydrate supplementation: A descriptive physiological profile of responders vs. nonresponders. The Journal of Strength & Conditioning Research. 2004 Aug 1;18(3):610-7.
Tarnopolsky M, Zimmer A, Paikin J, et al., Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS One. 2007;2(10):e991.
For local Townsville residents interested in FitGreyStrong’s Exercise Physiology services or exercise programs designed to improve muscular strength, physical function (how you move around during the day) and quality of life or programs to enhance athletic performance, contact FitGreyStrong@outlook.com or phone 0499 846 955 for a confidential discussion.
For other Australian residents or oversees readers interested in our services, please see here.
Disclaimer: All contents of the FitGreyStrong website/blog are provided for information and education purposes only. Those interested in making changes to their exercise, lifestyle, dietary, supplement or medication regimens should consult a relevantly qualified and competent health care professional. Those who decide to apply or implement any of the information, advice, and/or recommendations on this website do so knowingly and at their own risk. The owner and any contributors to this site accept no responsibility or liability whatsoever for any harm caused, real or imagined, from the use or distribution of information found at FitGreyStrong. Please leave this site immediately if you, the reader, find any of these conditions not acceptable.
© FitGreyStrong